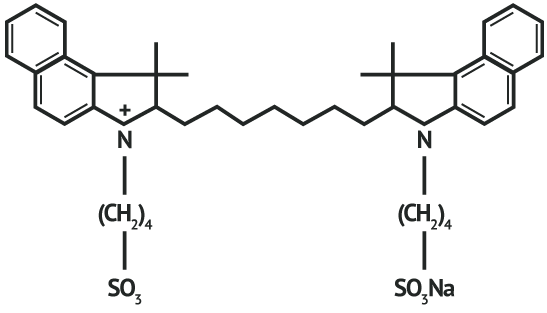
INDICATIONS AND USAGE: IC-Green® (indocyanine green for injection) is used for fluorescence imaging of vessels (micro- and macro-vasculature), blood flow and tissue perfusion before, during and after vascular, gastrointestinal, organ transplant, plastic, micro- and reconstructive surgeries, including general minimally invasive surgical procedures, in adults and pediatric patients aged 1 month and older; fluorescence imaging of extrahepatic biliary ducts in adults and pediatric patients aged 12 years and older; fluorescence imaging of lymph nodes and lymphatic vessels during lymphatic mapping in adults with cervical and uterine cancer and ophthalmic angiography in adults and pediatric patients. CONTRAINDICATIONS: ICG contains sodium iodide and should be used with caution in patients who have a history of allergy to iodides because of the risk of anaphylaxis. WARNINGS Deaths from anaphylaxis have been reported following ICG administration during cardiac catheterization. PRECAUTIONS: Drug Instability: ICG is unstable in aqueous solution and must be used within 6 hours. However, the dye is stable in plasma and whole blood so that samples obtained in discontinuous sampling techniques may be read hours later. Sterile techniques should be used in handling the dye solution as well as in the performance of the dilution curves. Drug Interactions: Preparations containing sodium bisulfite, including some heparin products reduce the absorption peak of ICG in blood and, therefore, should not be used as an anticoagulant for the collection of samples for analysis. Drug/ Laboratory Test Interactions: Radioactive iodine uptake studies should not be performed for at least a week following the use of ICG. Carcinogenesis, Mutagenesis, Impairment of Fertility: No studies have been performed to evaluate the carcinogenicity, mutagenicity, or impairment of fertility. Pregnancy and Nursing Mothers: Teratogenic Effects: Animal reproduction studies have not been conducted with ICG. It is also not known whether ICG can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. ICG should be given to a pregnant woman only if clearly indicated. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ICG is administered to a nursing woman. Pediatric Use: Safety and effectiveness in pediatric patients have been established. Geriatric Use: No overall differences in safety or effectiveness have been observed between elderly and younger patients. ADVERSE REACTIONS: Anaphylactic or urticarial reactions have been reported in patients with or without history of allergy to iodides. If such reactions occur, treat with the appropriate agents, e.g., epinephrine, antihistamines, and corticosteroids. OVERDOSAGE: There are no data available describing the signs, symptoms, or laboratory findings accompanying overdosage. The LD50 after I.V. administration ranges between 60 and 80 mg/kg in mice, 50 and 70 mg/kg in rats and 50 and 80 mg/kg in rabbits.