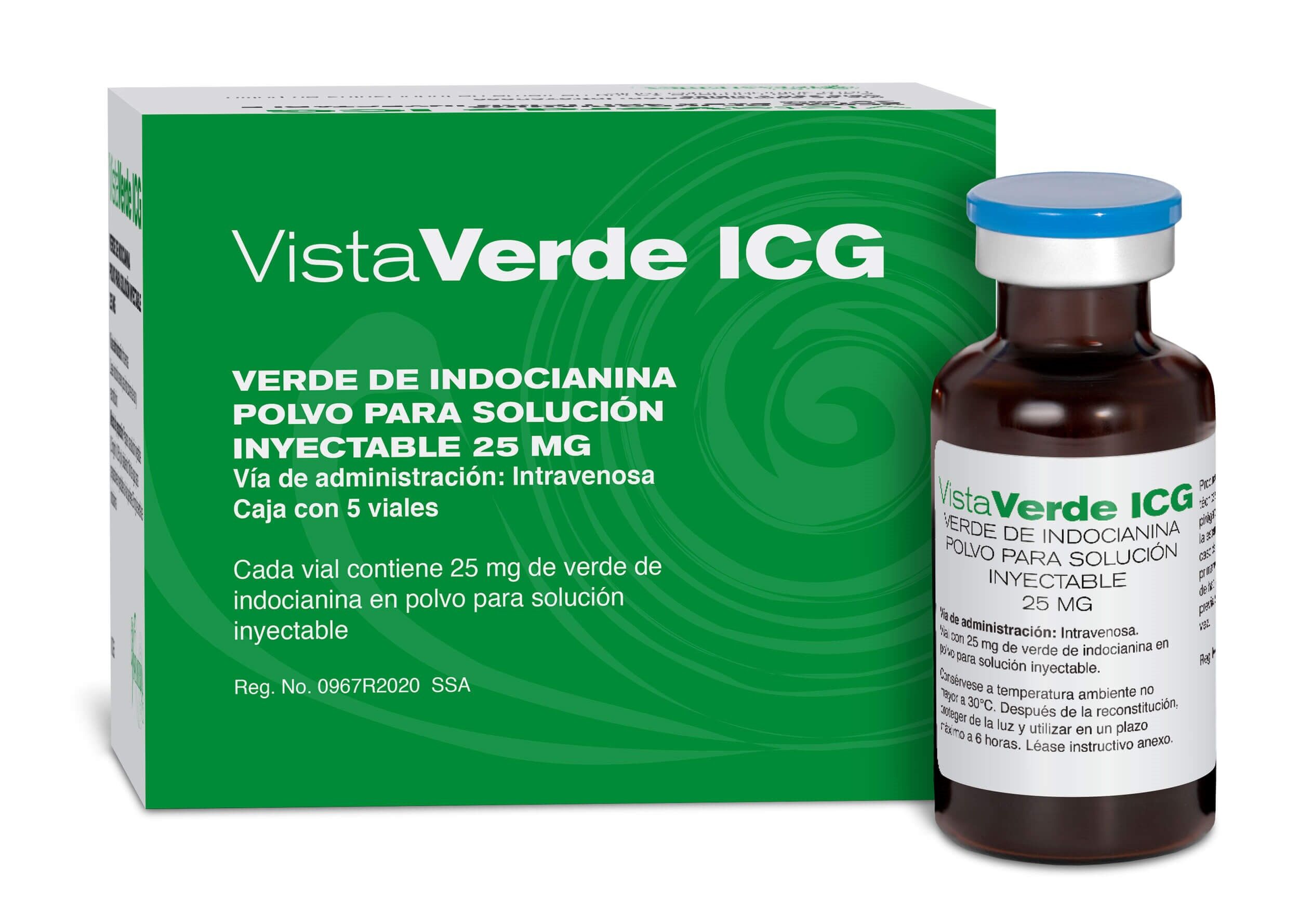
ICG now approved in Mexico – Vistamerica announced the approval of VistaVerde ICG in Mexico: Available on market in October 2020
Puerto Peñasco, Sonora. August 20, 2020. Vistamerica S de RL is proud to announce that VistaVerde ICG has been approved by the Federal Committee for Protection from Sanitary Risks (COFEPRIS) in Mexico. Vistamerica has exclusive distribution rights for Indocyanine Green (ICG) for the entire territory of Mexico, with plans set to enter the market in October 2020.
ICG, produced by Michigan based Diagnostic Green LLC, the leading provider of trusted high-quality fluorescence pharmaceutical products, is well known and accepted by physicians worldwide. Indicated for use in determining cardiac output, hepatic function and liver blood flow, and for ophthalmic angiography, ICG is well tolerated and has an excellent safety profile. It is routinely used by physicians during fluorescent guided surgery.
“We are delighted to engage with such a dedicated and enthusiastic company that will ensure ICG will be available to physicians throughout Mexico”, stated Ron Clarke, VP Business Development at Diagnostic Green.
Vistamerica cofounder and owner Catalina Markham similarly expressed, “We are excited to offer ICG in Mexico. It is quickly becoming the standard of care during fluorescent guided surgery and we are anxious to continue providing excellent service with cutting-edge products to benefit physicians and patients alike.”
For full prescribing details, contact Vistamerica.
Vistamerica S de RL Sinaloa Avenue No. 183-3, South Center, 83550, Puerto Peñasco, Sonora, Mexico.
Office: + 52-638-38-88668, Info@vistamerica.net.
VistaVerde
Verde de Indocianina
ICG ahora aprobado en México – Vistamerica anunció la aprobación de VistaVerde ICG en México: Disponible en el mercado en octubre del 2020
Puerto Peñasco, Sonora, 20 de agosto, 2020. Vistamerica S de RL (Vistamerica) se enorgullece de anunciar que VistaVerde ICG ha sido aprobado por la Comision Federal de Protección contra Riesgos Sanitarios (COFEPRIS) en México. Vistamerica tiene los derechos exclusivos de distribución de Indocyanine Green (ICG) para todo el territorio de México, con planes para ingresar al mercado en octubre del 2020.
ICG, es producido por Diagnostic Green LLC, con sede en Michigan, proveedor líder de productos farmacéuticos de fluorescencia de alta calidad de confianza, es bien conocido y aceptado por médicos de todo el mundo. Indicado para su uso en la determinación del gasto cardíaco, la función hepática y el flujo sanguíneo hepático, y para la angiografía oftálmica, el ICG se tolera bien y tiene un excelente perfil de seguridad. Los médicos lo utilizan habitualmente durante la cirugía guiada por fluorescencia.
“Estamos encantados de colaborar con una empresa tan dedicada y entusiasta que garantizará que ICG esté disponible para los médicos en todo México”, afirmó Ron Clarke, vicepresidente de desarrollo comercial de Diagnostic Green.
La cofundadora y propietaria de Vistamerica, Catalina Markham, expresó de manera similar: “Estamos muy contentos de ofrecer ICG en México. ICG se está convirtiendo rápidamente en el estándar de atención durante la cirugía guiada por fluorescencia y estamos ansiosos por continuar brindando un servicio excelente con productos de vanguardia para beneficiar a médicos y pacientes por igual”.
Para obtener detalles completos, comuníquese a Vistamerica.
Vistamerica S de RL Avenida Sinaloa No. 183-3, Centro Sur, 83550, Puerto Peñasco, Sonora, México.
Oficina: + 52-638-38-88668, Info@vistamerica.net.