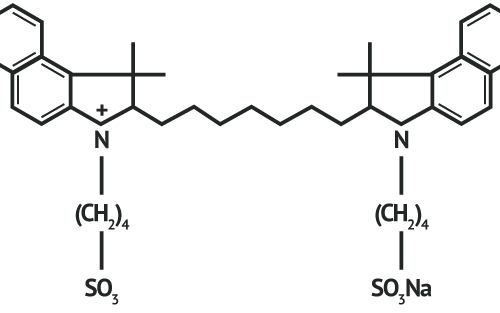
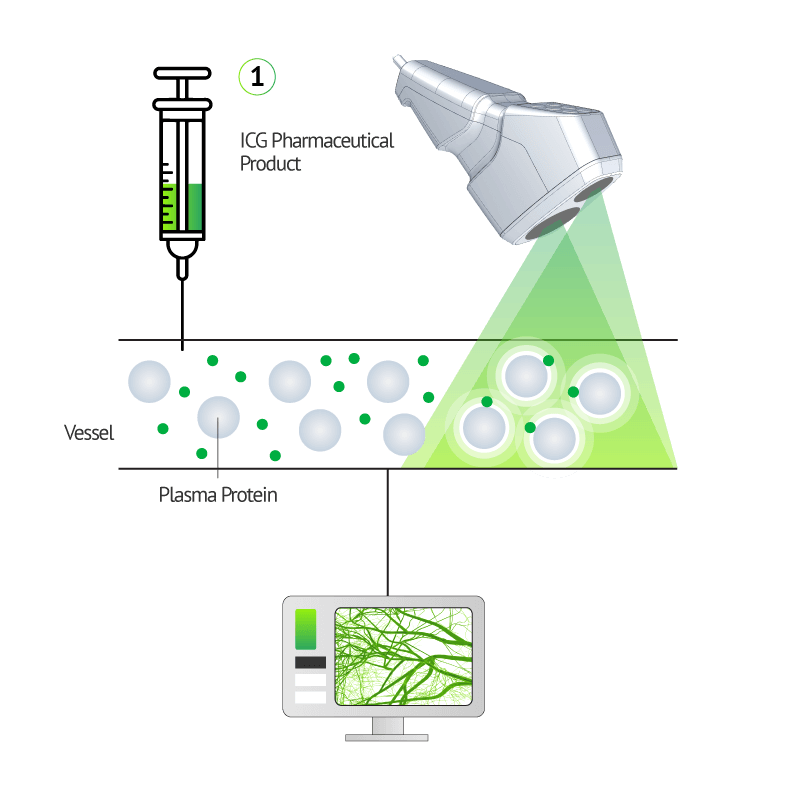
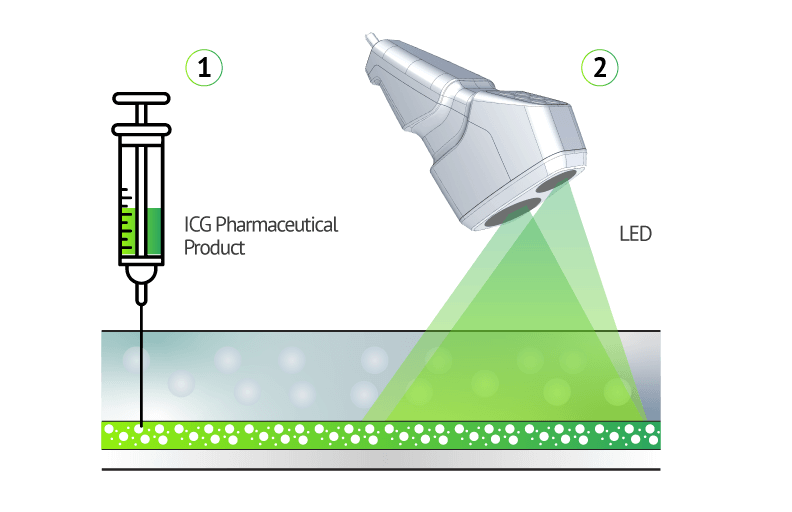
VERDYE™ 25 mg/50 mg. Active substance: indocyanine green, monosodium salt. Composition: 25 mg /50 mg powder for producing an injectable solution; 1 ml of the injectable solution contains 5 mg of the active substance. Other ingredients: none. Indications: diagnostic drug which is used to determine the effectiveness of the blood supply to the heart, brain and other organs and to one of the inner layers of the eye (choroid), as well as for monitoring the circulation of blood and determining liver function. Contraindications: hypersensitivity (allergy) to indocyanine green, sodium iodide or iodine; overactive thyroid or discrete benign thyroid tumours or if parts of your thyroid are no longer under hormonal influence. Do not use if you have ever had any side effects after receiving an ICG injection (risk of anaphylactic reaction). No use in premature babies and new-borns suffering from hyperbilirubinaemia. Side-effects: very rare (<1 in 10,000): nausea and hypersensitivity reactions (allergies); the possibility of an allergic reaction is greater in patients with extremely serious kidney failure. Possible symptoms: restlessness, hot flushes, nausea, itching, hives (skin rash), faster heartbeat, facial redness, swelling of the face (facial oedema), drop in blood pressure, shortness of breath, spasms of the bronchia and larynx, cardiac/circulatory arrest, death. Associated with the allergic reaction an increase of special white blood cells can occur. Prescription only. Diagnostic Green GmbH, Aschheim-Dornach, Germany, April, 2019.